BiTE® technology: designed to ENGAGE T CELLS TO FIGHT CANCER
Cytotoxic T cells play an important role in the body’s immune defense by identifying and eliminating cancer cells; however, cancer cells can develop mechanisms to evade T-cell recognition and destruction.1-3
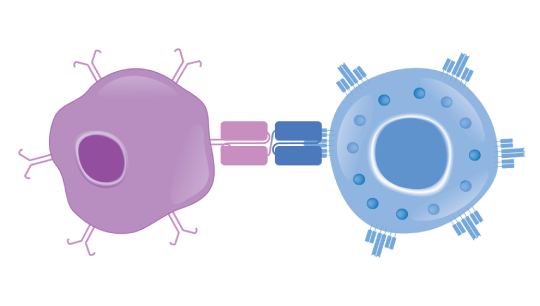
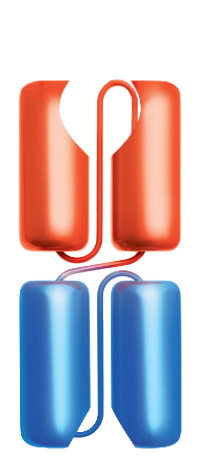
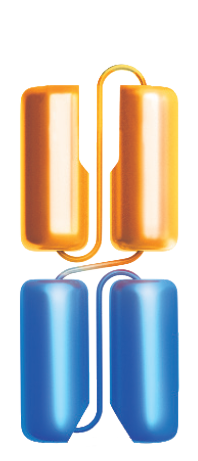
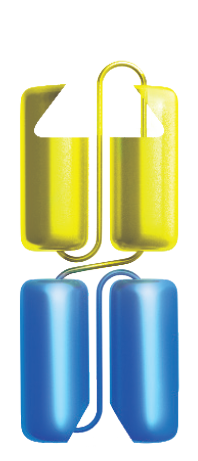
Bispecific T-cell Engager (BiTE®) technology is designed to overcome cancer cells’ evasion of the immune system by engaging patients’ own T cells to directly target cancer cells. BiTE® molecules are comprised of two flexibly linked, single-chain variable fragments, with one designed to bind specifically to a selected cell surface tumor-associated antigen and the other to bind CD3, a component of the T cell receptor found on the surface of T cells.1,4,5
Amgen has also advanced half-life extended BiTE® molecules that contain a silenced fragment crystallizable (Fc) domain.6-8 The Fc domain is designed to increase the amount of time that the half-life extended BiTE® molecule is in circulation and allow for less frequent administration.6,9
BiTE ® technology aims to provide a targeted immunotherapy approach in both hematologic malignancies and solid tumors.1
Search our clinical trials.
Visit our resources section for further information on modalities currently under investigation.
BiTE® technology is designed to fight cancer
1
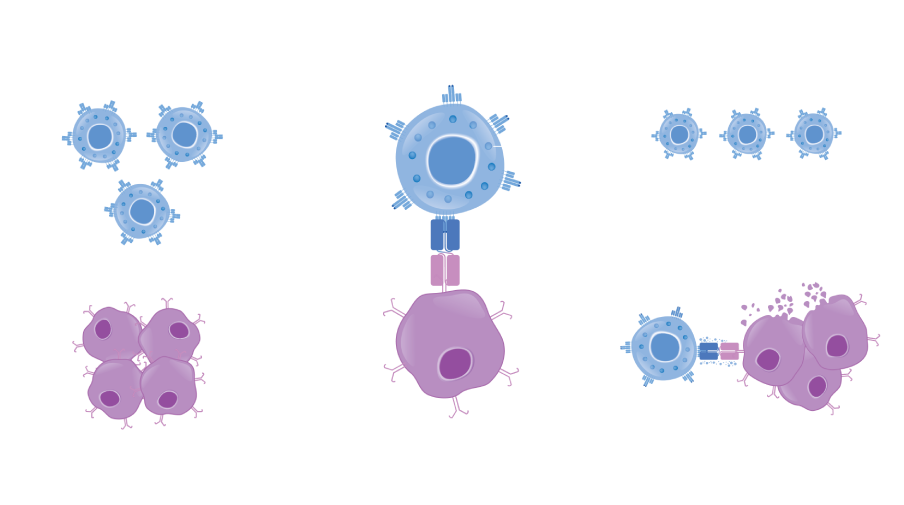
The BiTE® molecule is designed to recruit a T cell to bind to a cancer cell, leading to the formation of a cytolytic synapse1,10
2
The T cell becomes activated, releasing perforin and granzymes; fusion of perforin with the cancer cell membrane allows granzymes to enter the cancer cell to induce apoptosis10,11
3
T-cell activation also causes the release of cytokines and production of additional perforin and granzymes that may allow T cells to target surrounding cancer cells; this potentially results in the serial lysis of multiple cancer cells by a single T cell1,10,12
The BiTE® immuno-oncology platform offers versatility to potentially target any tumor-associated antigen
The BiTE® platform is being studied across a wide range of settings, including in patients with high and low tumor burden, rapidly progressing disease, or across different treatment lines.14-16
BiTE® molecules under clinical investigation include the following targets1,14:
*Half-life extended BiTE® platform.1
BiTE® molecules are designed to bring T-cell innovation to more patients
- Designed to target tumor-associated antigens14
- Investigated for use as monotherapies and in combination with other treatments2,11,16
The BiTE® platform is being investigated across a broad set of cancers1
The BiTE® immuno-oncology platform has been studied in thousands of patients, many of whom have been followed for up to 5 years.4,5
With the BiTE® immuno-oncology platform, Amgen is driven to push the boundaries of science for patients with cancer by:
- Leveraging innovative trial designs11
- Investigating clinically relevant endpoints and outcomes10,12,13
Amgen is pioneering BiTE® technology to advance the immuno-oncology field and bring new therapeutic approaches to patients
Features of the BiTE® platform
- Versatile options to target T cells to tumor cells1
- Potential for one or two target binding domains17
- Potential for high or low CD3 binding affinity8
- Different formats could allow administration by continuous intravenous infusion or by IV every two weeks6,9
Explore the resources below to learn more about the BiTE® Platform
ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; BiTE: Bispecific T-cell Engager; CD: cluster of differentiation; DLL3: delta-like protein 3; FLT3: FMS-like tyrosine kinase 3; GEJ: gastroesophageal junction; MUC17: mucin 17; PSMA: prostate-specific membrane antigen; SCLC: small cell lung cancer.
1. Baeuerle PA, Kufer P, Bargou R. Curr Opin Mol Ther. 2009;11(1):22-30. 2. Ferrone S, Whiteside TL. Surg Oncol Clin N Am. 2007;16(4):755-774. 3. Rabinovich GA, Gabrilovich D, Sotomayor EM. Annu Rev Immunol. 2007;25(4):267-296. 4. Frankel SR, Baeuerle PA. Curr Opin Chem Biol. 2013;17(3):385-392. 5. Yuraszeck T, Kasichayanula S, Benjamin JE. Clin Pharmacol Ther. 2017;101(5):634-645. 6. Weidle UH, Tiefenthaler G, Weiss EH, Georges G, Brinkmann U. Cancer Genomics Proteomics. 2013;10(1):1-18. 7. Trinklein ND, Pham D, Schellenberger U, et al. MAbs. 2019;11(4):639-652 8. Buelow B, Dalvi P, Dang K, et al. J Clin Oncol. 2021;39(15_suppl). doi:10.1200/JCO.2021.39.15_suppl.TPS5092. 9. Arvedson TL, Balazs M, Bogner P, et al. Cancer Res. 2017;77(suppl 13):Abstract 55. 10. Nagorsen D, Baeuerle PA. Exp Cell Res. 2011;317(9):1255-1260. 11. Baeuerle PA, Reinhardt C. Cancer Res. 2009;69(12):4941-4944. 12. Ross SL, Sherman M, McElroy PL, et al. PLoS One. 2017;12(8):e0183390. 13. Brischwein K, Schlereth B, Guller B, et al. Mol Immunol. 2006;43(8):1129-1143. 14. Ok CY, Young KH. J Hematol Oncol. 2017;10(1):103. 15. Baudino TA. Curr Drug Discov Technol. 2015;12(1):3-20. 16. Shekarian T, Valsesia-Wittmann S, Caux C, Marabelle A. Mutagenesis. 2015;30(2):205-211.17. Deshaies RJ. Nature. 2020;580(7803):329-338.