IMMUNE THROMBOCYTOPENIA (ITP)
ITP IS A RARE AUTOIMMUNE DISORDER CHARACTERIZED BY DECREASED PLATELET COUNTS AND INCREASED RISK OF BLEEDING1
- Primary ITP is characterized by platelet counts <100 x 109/L in the absence of other identifiable causes of thrombocytopenia1
- ITP affects all ages and genders, with the highest incidence in children ( <18 years old) and older adults (>60 years)2,3
- The main clinical burden on ITP is the increased risk of bleeding. The presentation of bleeding symptoms varies from patient to patient, from mild bruising tendency to major bleeding1:
- Patients can present with petechiae, purpura, mucosal bleeding, epistaxis, internal bleeding, and intracranial hemorrhage4,5
- Some patients are asymptomatic and diagnosed by chance during work-up for other medical issues4,6
- Severe bleeding occurs in ~10% of adults and 20% of children with newly diagnosed or chronic ITP7
- Intracranial hemorrhage is a rare but serious complication in ITP with incidence of 1–1.8% in adults and 0.6% in children8
- Platelet counts <20 x 1012/L are frequently associated with severe bleeding events.9 Newly diagnosed, elderly, and patients with prior bleeding events are at higher risk5
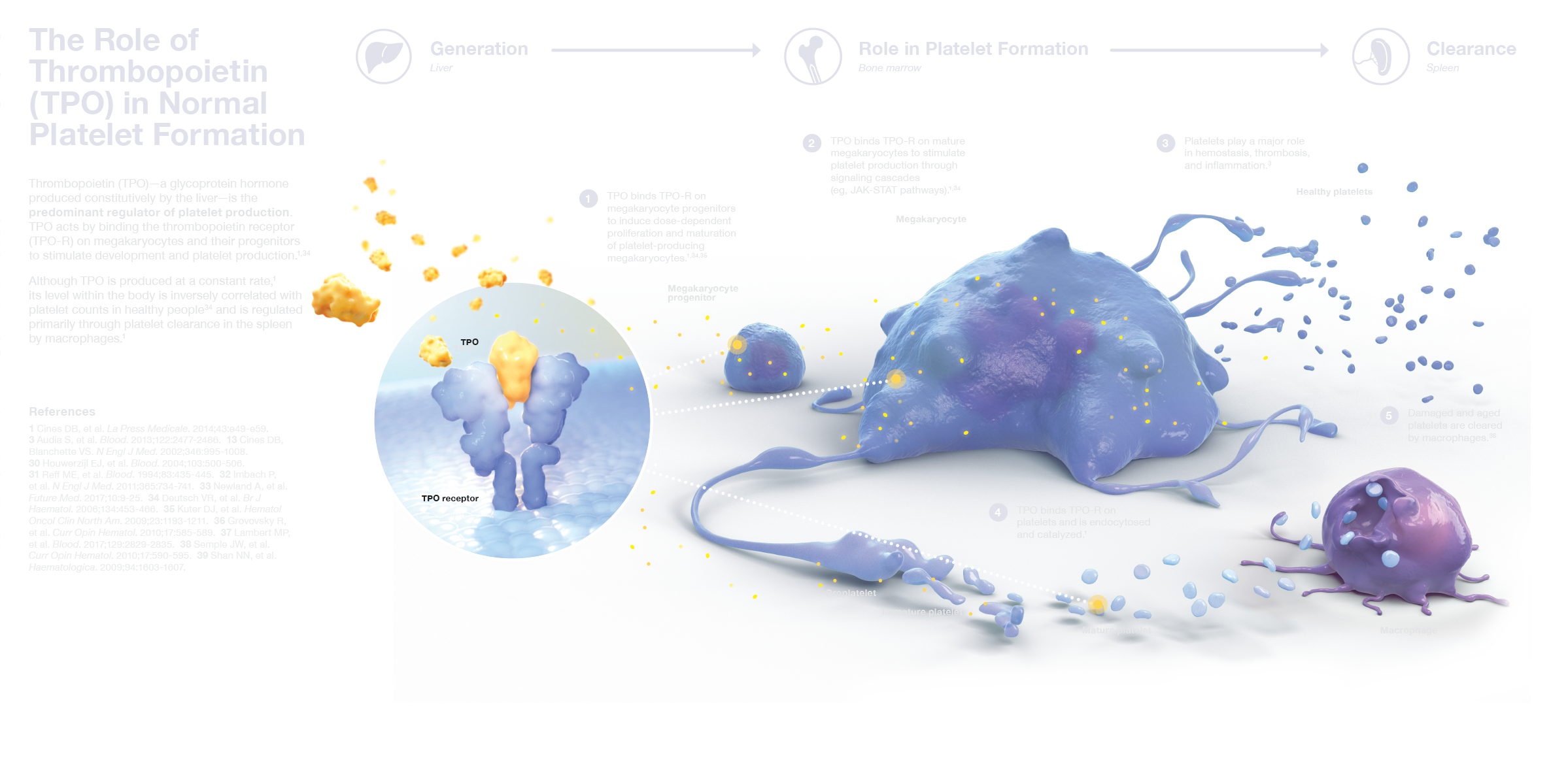
NORMAL PLATELET PHYSIOLOGY: THROMBOPOIETIN IS THE PRIMARY DRIVER OF PLATELET PRODUCTION10
- Platelets are produced by megakaryocytes in the bone marrow in response to thrombopoietin (TPO), the predominant regulator of platelet production10
- TPO acts by binding the thrombopoietin receptor (TPO-R) on megakaryocytes and their progenitors to stimulate their development and platelet production10,11
ITP pathophysiology:
accelerated platelet destruction and impaired platelet production
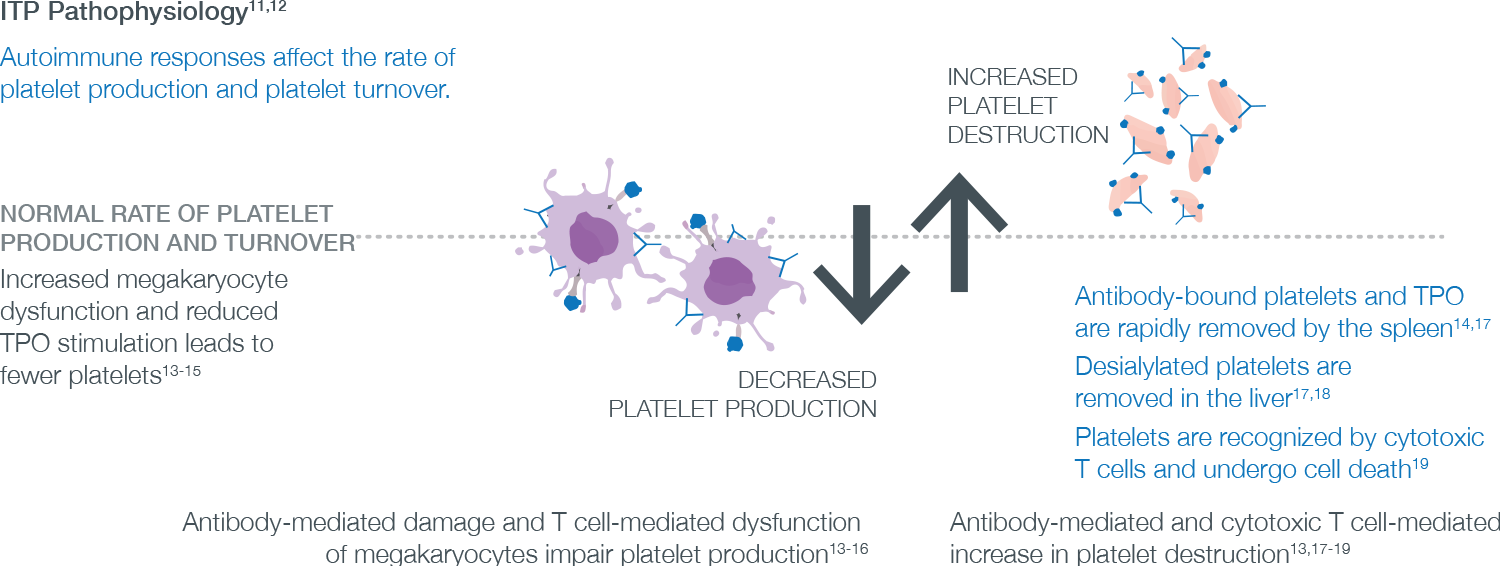
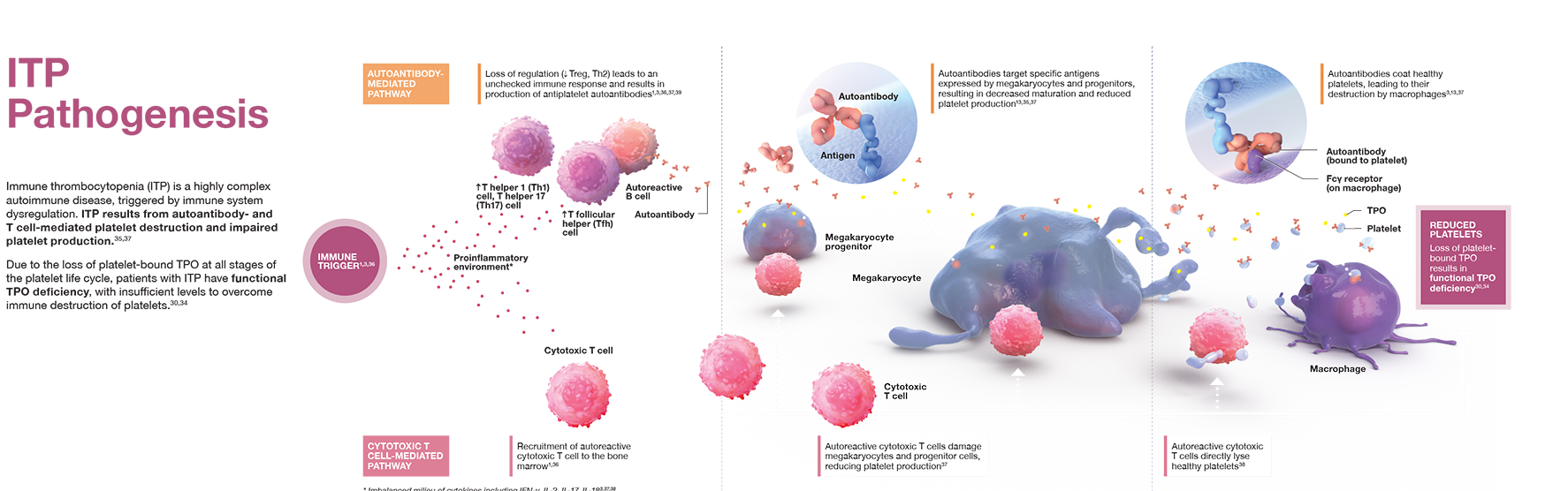
- ITP is a complex and heterogeneous disease triggered by immune system dysregulation, resulting in increased platelet destruction and impaired platelet production12
- Autoantibody-mediated pathway
- Loss of regulation (decrease Treg, increase Th1/Th2 ratio) leads to an unchecked immune response and results in production of antiplatelet autoantibodies11,13,14
- Autoantibodies bind to healthy platelets, resulting in their sequestration and destruction by macrophages in the spleen4
- Autoantibodies target specific antigens expressed by megakaryocytes and progenitors, resulting in decreased maturation and impaired platelet production4,15
- Cytotoxic T cell mediated pathway
- Autoreactive cytotoxic T cells damage megakaryocytes and progenitor cells, and directly lyse healthy platelets13
- Due to the loss of platelet-bound TPO, patients with ITP have functional TPO deficiency, with insufficient levels to overcome immune destruction of platelets10,16
Treatment strategies for ITP
The primary goal of treatment is to sustain platelet counts that are associated with adequate hemostasis, and reduce bleeding risk with minimal side effects. Treatments should be tailored to individual patients, taking into account the patient’s age, severity of illness, bleeding risk, comorbidities, lifestyle considerations, and careful evaluation of benefit/risk profile of each therapy.1,17 Treatment options may include the following:
Reduce platelet destruction
| Immunoglobulin (IVig, Anti-D)2 | Blocks Fcγ receptors on macrophages to prevent their recognition of autoantibody-coated platelets |
| Immunosuppression (corticosteroids)2 | Suppresses B and T cell-mediated autoantibody production, and impairs the ability of macrophages within the bone marrow to destroy platelets |
| Inhibition of B cells2,18 | Targets CD20+ B cells to lower production of antiplatelet autoantibodies and block macrophage Fcγ receptors |
| Non-specific immunosuppression (eg, azathioprine, cyclosporine)2 | Nonspecifically inhibits T cells to interfere with immune activation |
| Splenectomy2 | Removes the main site of platelet destruction (fewer macrophages are available to clear antibody-coated platelets) |
| Syk inhibition19 | Impairs the FcR signaling pathway involved in phagocytosis and autoantibody-coated platelets |
STIMULATE PLATELET PRODUCTION
| TPO-R agonists20 | Bind TPO-R to stimulate platelet production thereby raising platelet counts to outpace excess platelet destruction |
For a range of additional materials about ITP, visit our resources or tap on any thumbnail below to view.



Treg: regulatory T cell; IVig: intravenous immune globulin; Fcy: Fc gamma; FCR: Fc receptor.
References:
1. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. 2. Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 2009;145(2):235-244. 3. Moulis G, Palmaro A, Montastruc JL, Godeau B, Lapeyre-Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood. 2014;124(22):3308-3315. 4. Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346(13):995-1008. 5. Altomare I, Cetin K, Wetten S, Wasser JS. Rate of bleeding-related episodes in adult patients with primary immune thrombocytopenia: a retrospective cohort study using a large administrative medical claims database in the US. Clin Epidemiol. 2016;8:231-239. 6. Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3):495-520. 7. Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13(3):457-464. 8. Kühne T, Berchtold W, Michaels LA, et al. Newly diagnosed immune thrombocytopenia in children and adults: a comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. Haematologica. 2011;96(12):1831-1837. 9. Cines DB, Bussel JB. How I treat idiopathic thrombocytopenia purpura (ITP). Blood. 2005;106(7):2244-2251. 10. Deutsch VR, Tomer Al. Megakaryocycte development and platelet production. Br J Haematol. 2006;134(5):453-466. 11. Cines DB, Cuker A, Semple JW. Pathogenesis of immune thrombocytopenia. La Press Med. 2014;43(4 pt 2):e49-e59. doi:10.1016/j.lpm.2014.01.010. 12. Cines DB, Bussel JB, Liebman HA, Prak ETL. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511-6521. 13. Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129(21):2829-2835. 14. Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmunity Rev. 2017;16(6):620-632. 15. Kuter DJ, Gernsheimer TB. Thrombopoietin and platelet production in chronic immune thrombocytopenia. Hematol Oncol Clin North Am. 2009;23(6):1193-1211. 16. Houwerzijl EJ, Blom NR, van der Want JJL, et al. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenia purpura. Blood. 2004;103(2):500-506. 17. Matzdorff A, Meyer O, Ostermann H, et al. Immune thrombocytopenia – current diagnostics and therapy: recommendations of a Joint Working Group of DGHO, ÖGHO, SGH, GPOH, and DGTI. Oncol Res Treat. 2018;41(suppl 5):1-30. 18. Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435-445. 19. Newland A, Lee, EJ, McDonald V, Bussel JB. Fostamatinib for persistent/chronic adult immune thrombocytopenia. Immunotherapy. 2018;10(1):9-25. 20. Imbach P, Crowther M. Thrombopoietin-receptor agonists for primary immune thrombocytopenia. N Engl J Med. 2011;365(8):734-741.

